Abstract
While much progress has been made in the treatment of B-cell acute lymphoblastic leukemia (ALL), relapsed and refractory disease is an ongoing problem. Even though the development of CD19 CAR-T cell therapy and CD19 ADCs/bispecific based therapeutics have improved the response rates significantly; the emergence of resistant cell populations can lead to relapsed disease. Evidence suggests that resistance can be facilitated by the decreased expression of CD19 on malignant cells leading to loss of CAR-T efficacy. Modeling adaptive resistance is important in elucidating the cellular and molecular mechanism of resistance and in uncovering newer therapeutic targets in resistant clonal populations.
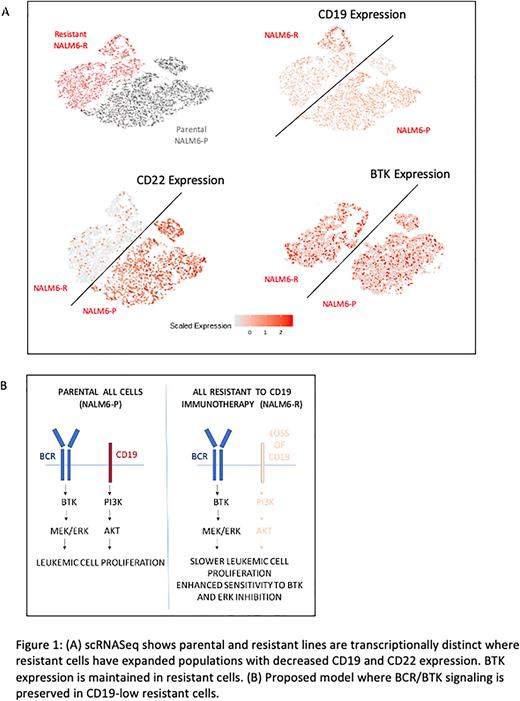
We used an immunotoxin comprising a CD19 antibody conjugated to recombinant ricin toxin (HD37-dgRTA) to build a model of adaptive resistance to CD19 based immunotherapy. B-ALL cell lines (NALM-6 and REH) were grown in presence of escalating doses of the CD19-immunotoxin and led to emergence of resistant clones in vitro with decreased expression of CD19 by FACS analysis. The transcriptomic profiles of the parental and resistant cells showed distinct populations with differential gene expression. Specifically, the slower growing resistant cells had decreased expression of CD22 in addition to CD19, as well as decreased expression of other mature B cell lineage differentiation markers. Analysis of patient B-ALL samples pre and post CD19 CAR-T also show a decrease in CD22 expression with interval loss of CD19. Interestingly, BTK expression was maintained in the resistant cells. Single cell RNA-seq analysis demonstrated that a small clone of pre-existing CD19-low cells were present in parental cell lines, that expanded significantly upon CD19-immunotxin exposure. CyTOF analysis was conducted that showed loss of CD19 expression at the protein level, with preserved expression of B cell receptor associated p-PLC-gamma and p-CREB signals. Preserved BCR expression with reduced CD19 expression was also seen in primary patient sample that acquired resistance to CD19 CAR-T therapy.
Within the cell signaling machinery of B-cells, CD19 and the B cell receptor (BCR) can both lead to stimulatory signals that promote cell proliferation leading to downstream MEK pathway activation. BCR uses the BTK driven pathways while CD19 is uses PI3K and AKT to induce proliferation. We evaluated the sensitivity of parental and resistant NALM-6 cells to BTK and MEK inhibitors and observed that the resistant (CD19-low) cells were more sensitive to both inhibitors. Immunoblotting showed that loss of CD19 (in CRISPR mediated CD19 KO Raji cells) is associated with reduced MEK pathway activation, that can be significantly abrogated by BTK inhibitors. Lastly, primary human samples from B-ALL patients that were resistant to CD19 immunotherapies demonstrated sensitivity to BTK inhibitors in vitro.
These data demonstrate that an in-vitro model of adaptive resistance to CD19 immunotherapies can be created that shows that small numbers of pre-existing CD19-low clones expand during therapy resistance. The resistant cells revert to a less differentiated transcriptional program with concomitant decrease in CD22 also, an observation that we validated in patient samples. We also demonstrate that BCR/BTK signaling is preserved in resistant cells that makes them vulnerable to BTK inhibitors. Inhibiting BTK/MEK signaling could be an avenue for targeting CD19 CAR-T resistant cell populations.
Disclosures
Sica:AstraZeneca: Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Kite Pharma: Research Funding; PER Physician's Education Review.: Consultancy, Honoraria; Bristol Myers Squibb Foundation: Research Funding; Miragen: Consultancy, Honoraria; Curios: Honoraria. Schinke:Janssen: Honoraria. Janakiram:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Barta:Affimed: Consultancy; Daiichi Sankyo: Consultancy; Kyowa Kirin: Consultancy, Honoraria; Seagen: Honoraria; Janssen: Other: Independent Data Monitoring Committee member; Acrotech: Honoraria. Shastri:Janssen: Consultancy; Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; NACE: Honoraria; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Brody:Genentech: Research Funding; Gilead/Kite: Research Funding; Merck: Research Funding; SeaGen, Roche, Genentech, Merck, ADC Therapeutics, Epizyme, Gilead, Kite, Astrazeneca: Research Funding; ADC Therapeutics: Consultancy; Epizyme: Consultancy; Seagen: Consultancy; BMS: Research Funding. Verma:Stelexis Therapeutics: Current equity holder in publicly-traded company; Throws Exception: Current equity holder in publicly-traded company; BMS: Research Funding; GlaxoSmithKline: Research Funding; Jannsen: Research Funding; Eli Lilly and Company: Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Curis: Research Funding; Stelexis Therapeutics: Consultancy; Acceleron Pharma: Consultancy; MedPacto: Research Funding; Incyte: Research Funding.
OffLabel Disclosure:
Ibrutinib is a small molecule inhibitor of Bruton's tyrosine kinase. Trametinib is a small molecule inhibitor of MEK1 and MEK2.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal